NPA: ‘Notable number’ of anticoagulant dispensing errors in 3 months
There were a “notable number” of errors involving direct oral anticoagulants (DOACs) in the three months to June, the NPA's former medication safety officer (MSO) has said.
Rosuvastatin and rivaroxaban dispensed in place of each other was the most common type of “wrong drug” patient safety incident reported to the National Pharmacy Association (NPA) in April-June, Leyla Hannbeck revealed in her final MSO report, published last week (July 26).
“As anticoagulants, these medicines are considered high risk,” she added.
Ms Hannbeck recommended pharmacies separate high-risk medicines in a dedicated anticoagulant area of the dispensary to help reduce picking errors, as well as ensure patients prescribed DOACs are provided with educational materials when treatment is initiated.
Dispensing wrong strength of pregabalin
Medication errors – including the wrong strength, drug or formulation being dispensed – accounted for 67% of all errors reported, with the wrong strength dispensed on 27% of these occasions.
Despite the rescheduling of gabapentin and pregabalin as class C controlled substances in April, 5% of all “wrong strength errors” made involved one of these drugs, Ms Hannbeck said.
The wrong strength of inhaler was also an issue – accounting for 4.5% of “wrong strength errors” – including “multiple errors” involving Fostair 100/6 or Fostair 200/6 inhalers.
Understaffing and time pressures
“Work and environment factors” continued to be the main contributing factor to patient safety incidents, accounting for 34% of reports. Pharmacies continued to document time pressures, understaffing and cluttered or poorly organised working environments as contributing factors, Ms Hannbeck said.
In one example highlighted in the report, work and environmental factors contributed to an incident involving an individual on long-term oral steroids.
“A patient was issued a prescription for prednisolone tablets 5mg, at a reducing dose. The pharmacy dispensed prednisolone tablets 1mg in a plain white container. The box was labelled correctly against the prescription with the dosage instructions: ‘four tablets daily – reducing by one tablet every week’,” Ms Hannbeck explained.
The patient took a “significantly lower” dose for three weeks and experienced nausea and vomiting. After three ambulance calls, the patient was admitted to hospital for three days.
Before dispensing steroids, pharmacy staff should confirm a steroid dose, check the patient knows how to take steroids, issue a steroid card and advise patients to seek medical attention if they feel unwell, she added.
Calculation error
Another “common error theme” highlighted in the report involved a miscalculation of ranitidine dose for a child.
“A prescription was received for ranitidine 150mg/10ml (75mg/5ml) for a child with a dose instruction of 25mg twice a day. The dispenser changed the dose instructions to a volume in ml to assist the carer,” Ms Hannbeck explained.
The dispenser misread the dose as 25ml and a label was produced instructing the patient to take approximately 15 times the dose prescribed: ‘five 5ml spoonfuls’.
Doses prescribed by patient weight should be confirmed with the patient or representative and instructions on how to give each dose should be specific, Ms Hannbeck advised. Those administering medicine should be encouraged to use “oral syringes or other measuring devices to facilitate accurate does administration of liquid formulations”.
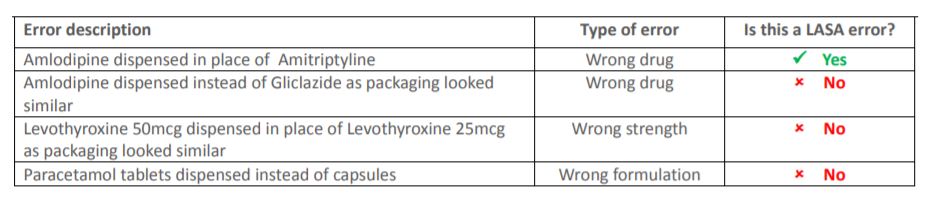
LASA errors and decreasing reports
A total of 29% of all incidents were reported to the NPA as look-alike, sound-alike errors. However, 32% of these reports were in fact wrong strength, wrong formulation or errors involving similar packaging, Ms Hannbeck said.
Ms Hannbeck’s guide below sets out what is classed as a look-alike, sound-alike error.
The majority of all incident reports – 56% – continued to involve no harm to the patient, while 29% were reported as “near misses”.
The number of reports received by the NPA fell by 19% in April-June. Ms Hannbeck suggested this was because it followed a “particularly high number of reports” received in February, as this was the final review point of the 2018-19 Quality Payments Scheme.





Have you ever spotted an anticoagulant dispensing error in your pharmacy?